
Previous
Good Health for 2014: Five Ways to Protect Against Fatty Liver

Next
7 Ways to Stop Liver Scar Progression
Improved Liver Evaluation Test Available in the U.S.
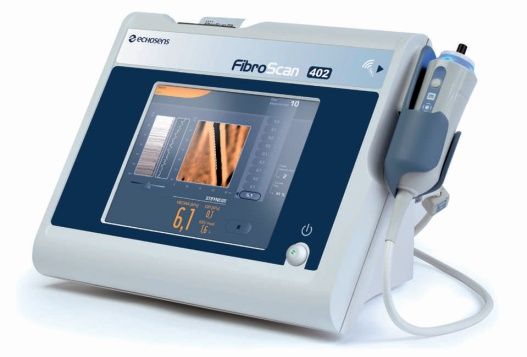
The FDA gave FibroScan® the green light, an approval projected to drastically improve liver fibrosis testing.
Liver biopsy stood its ground as the gold standard for assessing liver health for decades, but less-invasive options are available. As of 2013, America joined over 70 other countries in approving the use of FibroScan® to determine the presence and extent of liver damage. If you have reason for determining or monitoring liver fibrosis, make sure you know the pros and cons of the FibroScan® test.
Liver Biopsy
A liver biopsy is a diagnostic procedure where a needle is inserted into the liver to obtain a small amount of liver tissue. That sample is then examined under a microscope to determine the presence of and the degree of liver scarring (fibrosis). Although it is used for various other liver ailments, a liver biopsy is especially useful to evaluate patients with viral Hepatitis C or Hepatitis B in staging the disease and determining whether or not the person is a candidate for treatment. Despite its long reign as being the preferred liver diagnostic method, liver biopsy has a long list of disadvantages.
A liver biopsy:
- is an invasive test because a hollow needle must pierce the skin and liver to retrieve a tissue sample.
- requires hospitalization for at least several hours.
- involves sedation.
- is expensive.
- carries some risk such as pain and bleeding.
- does not provide immediate results because it requires the sample be sent to a lab.
- can yield sampling error, as the portion removed may not represent the entire liver.
- is open to different interpretation by different pathologists.
FibroScan®
Also called transient elastography, FibroScan® assesses liver stiffness without being invasive. Transient elastography is feasible because an increase in liver scarring increases tissue rigidity. By sending sound waves into the liver causing it to vibrate, the FibroScan® measures the shear wave velocity. This is accomplished by passing a 50-MHz wave into the liver from a small transducer on the end of an ultrasound probe. Although approved by the U.S. Food and Drug Administration (FDA) on April 5, 2013, FibroScan® has been the liver diagnostic tool of choice elsewhere for up to a decade.
Currently available in 70 countries, FibroScan®:
- was introduced in the European market in 2003.
- received market clearance in China in 2008.
- received market clearance in Canada in 2009.
- received market clearance in Brazil in 2010.
- received market clearance in Japan in 2011.
Some advantages of FibroScan® include:
- It is not painful and non-invasive, with the patient feeling just a slight vibration on their skin.
- Sedation is not necessary.
- The test takes less than ten minutes.
- It is much less expensive than liver biopsy.
- There have not been any side effects associated with FibroScan®.
- The results are instantaneous.
- The procedure can be safely repeated as often as needed.
- FibroScan® is very accurate for staging those with no or minimal fibrosis and for advanced fibrosis or cirrhosis.
However, FibroScan® does have some limitations:
- It is not as accurate as needle biopsy for those with mid-level liver disease.
- It is not advised for patients with ascites, morbid obesity and/or large amounts of chest wall adiposity.
Other Non-Invasive Liver Diagnostic Tests
FibroScan® is just one type of non-invasive liver diagnostic test. Both radiologic and serum biomarker tests can provide accurate liver stiffness results:
- Radiologic methods – Besides the FibroScan®, the most common radiologic liver stiffness test is magnetic resonance (MR) elastography. MR elastography delivers comparably accurate results, but it requires an MR image scan which is both costly and time-consuming. Unlike the FibroScan®, MR elastrography involves a separate appointment due to the specialized equipment needed.
- Serum Biomarker Tests – These blood tests measure biomarkers that are associated with fibrosis. The most common serum tests for staging liver fibrosis are HepaScore, FibroSure, the FIB-4 index and the European Liver Fibrosis test. Similarly, serum biomarker tests have demonstrated a comparable accuracy to the MR elastrography and the FibroScan®, but the blood samples usually have to be sent to a lab. Thus, the results are not instantaneous.
Experts believe that serum biomarker tests and radiologic tests are not mutually exclusive; as many guidelines currently recommend combining the two types of testing. Admittedly, there are a few limitations to FibroScan® testing. However, the cost, time and comfort benefits of this non-invasive liver test are sure to transform the practice of hepatology – an advancement that U.S. clinicians can finally utilize.
http://www.echosens.com/Public-EN/diagnostic.html, What Diagnostic Methods are Available? Retrieved January 13, 2014. Echosens. 2013
http://hepatitiscnewdrugs.blogspot.com/2013/10/newly-approved-ultrasound-device.html, Newly Approved Ultrasound Device Eliminates Risks and Pain of Liver Biopsy, Retrieved December 15, 2013, PRNewswire, 2013.
http://www.liver.ca/liver-disease/diagnosing-liver-disease/liver-biopsy.aspx, Liver Biopsy, Retrieved December 15, 2013, Canadian Liver Foundation, 2013.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3594956/, Fibroscan (Transient Elastography) for the Measurement of Liver Fibrosis, Nezam H. Afdhal, MD, Retrieved December 10, 2013, Journal of Gastroenterology and Hepatology, September 2012.
http://www.prnewswire.com/news-releases/fda-approves-fibroscan-for-non-invasive-liver-diagnosis-203186961.html, FDA Approves Fibroscan® for Non-Invasive Liver Diagnosis, Retrieved December 10, 2013, PR Newswire Association, LLC, 2013.
http://www.wwlp.com/news/health/new-liver-scan, Dr. Frank McGeorge, Retrieved December 10, 2013, New Liver Scan, LIN Television Corporation, 2013.






Where are Fibroscans machines located in Florida? Are there any in Brevard County, FL? Hate to have to travel to Miami for a 15 min. test, that is a 6 hr drive round trip for me.
MOST Insurance companies are NOT PAYING for this and it is expensive! $300 to $350 out of your pocket. And not surprisingly, Hepatologists are ordering this exam (when not really indicated, like no other indications of Fibrosis) because they invested in diagnostic facilities that own these ….. i.e. Kickbacks! The Liver, Kidneys, heart and other like “soft tissue” organs are especially receptive to fibrosis diagnosis with far cheaper Ultrasound which the Insurance plans WILL pay for! Unless you are already experiencing Liver cirrhosis or fibrosis indications that call for a Biopsy, you DON’T NEED this exam and don’t be pushed into it.
Keith B Ph.D.
Keith , you being a researcher and your wife begin HepB positive what is your take on supplements i.e. Are they helpful or harmful also are ther some that you have tried before, secondly does pain around your liver area always mean that you have liver damage
I can’t say all supplements are harmful, other than giving a person a false sense of doing something to cure or control Hepatitis. Absolutely none do, including “Eastern” herbal quack medicine. There are of course SOME vitamin supplements that help “improve” your body’s lack of intake, or self manufacture of vitamins thru foods or exposure to sun light. HepB is a virus, plain and simple and there is no evidence that any supplement or herbal concoction controls or eliminates any virus as virulent as Hepatitis. A best example is Taiwan, where the majority of the population has chronic HepB. Until recent years the government health services looked the other way to Herbal HepB and C treatments and doing little to push the population towards established western medications to treat Hepatitis; consequently millions have died (including my wife’s father of Liver Cancer). Now the Taiwanese government is forcing treatments with western medications and refusing to pay for Herbal and supplement treatments. The Chronic rates are falling. Your best armament to fight Hepatitis is get on a medication program that reduces the active viral count, eat well (foods) avoid alcohols and any intakes that is hard on your liver and reduces your bodies ability to remain in top performance. Also have an annual Liver Ultrasound to monitor it’s condition and it’s covered under all insurance programs now. Like HepC, a cure for HepB is within reach, perhaps only a couple years.
Keith, are you saying that an Ultrasound will detect fibrosis?
Of course it will! Just as Ultrasound can clearly display fibrocistic disease of the breast or any organ.