Clinical Studies on Silybin Phytosome
The clinical studies included in this section attest to both the safety and effectiveness of Silybin Phytosome, the active ingredient in Maximum Milk Thistle® and UltraThistle®.
The reason these studies were originally conducted was to have Silybin Phytosome introduced as a pharmaceutical drug in Europe. Unlike here in the U.S., European doctors take botanical medicine very seriously. Physicians prescribe herbal preparations on a regular basis for specific conditions and diseases.
-
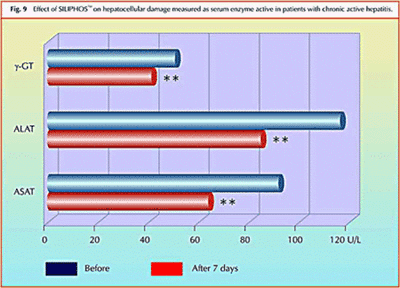
Effect of Silybin Phytosome on chronic active hepatitis:
Study shows significant reduction in elevated liver enzymes (and therefore hepatocellular necrosis) in just one week of treatment with Silybin Phytosome.
-
Therapeutic effect of Silybin Phytosome in chronic liver disease:
A small study of eight human subjects with chronic active hepatitis. This study shows significant benefits of Silybin Phytosome after just two months treatment.
-
Direct comparison of Silybin Phytosome to standardized milk thistle extract:
Study shows nearly 10x the bioavailability of Silybin Phytosome over the world’s best selling standardized extract.
-
Useful dosages of Silybin Phytosome:
Shows definite advantages of Silybin Phytosome at dosages ranging from 160mg to 360mg (measured as silybin). Dose/effect relationship is also demonstrated with the highest dosage tested getting the most dramatic results.
-
Considerably greater bioavailability of silybin as a component of Silybin Phytosome:
Laboratory study shows that binding silybin with phosphatidylcholine on a molecular level dramatically improves its bioavailablity on a cellular level.
-
Increased oral bioavailability of Silybin Phytosome in humans:
Human study compares Silybin Phytosome and the worlds best selling standardized milk thistle extract and shows how much better the results are with Silybin Phytosome.
-
Effect of Silybin Phytosome on cirrhotic patients:
Study shows that Silybin Phytosome does not significantly differ in its safe and beneficial activity in patients with cirrhosis vs. healthy control subjects.
-
Liver damage control properties of Silybin Phytosome:
Silybin Phytosome shows significant protective activity against liver damage. This study tested Silybin Phytosome protection against a variety of toxins.
-
Free radical scavenging properties of Silybin Phytosome:
Study shows the capability of Silybin Phytosome to scavenge free radicals and inhibit lipid peroxidation. It also demonstrates significantly higher blood plasma levels of silybin when administered as a component of Silybin Phytosome rather than in its unbound form.
-
Effect of silimarin on lipid peroxidation:
Study shows milk thistle extract (silymarin) to be a helpful antioxidant and silybin to be the most valuable constituent of silymarin.
-
Silybin Phytosome counteracts hepatotoxic effects:
Study shows Silybin Phytosome protects liver cells against free-radical mediated toxic liver injury.
-
Antioxidant activity of Silybin Phytosome against alcohol:
Study shows how Silybin Phytosome is dramatically more effective than pure silybin alone. Concludes Silybin Phytosome may be useful in counteracting damage caused by alcohol intake.
-
Tolerablility and effectiveness of Silybin Phytosome:
Study shows high dose tolerability of Silybin Phytosome as well as its increased effectiveness over conventional standardized milk thistle extract.
-
Liver protection potential of Silybin Phytosome:
Study shows the antioxidant and free radical scavenging effect of Silybin Phytosome.
-
Comparative bioavailability of Silybin Phytosome vs. silybin:
Study shows exactly how much better silybin is absorbed when combined on a molecular level with phosphatidylcholine (a patented process resulting in Silybin Phytosome).
-
Liver protective activity:
Shows Silybin Phytosome is more effective than its constituents, silybin and phosphatidylcholine, alone. The effectiveness of Silybin Phytosome is considerably greater than the sum of its parts.
-
Silymarin treatment of viral hepatitis: a systematic review (PDF)
Shows silymarin compounds likely decrease serum transaminases in patients with chronic viral hepatitis, but do not appear to affect viral load or liver histology.
Clinical Study – Effect of Silybin Phytosome on chronic active hepatitis
A pilot study on the liver protective effect of silybinphosphatidylcholine complex (Silybin Phytosome) in chronic active hepatitis
G. Buzzelli, S1., Moscarella, A. 1, Giusti, A. 1, Duchini, C. 1, Marena and M. Lampertico 21
Instituto di Clinica Medica , Universita Degli Studi di Firenze, Firenze and 2Medical Department, Inverni Dell Beffa, Milan ItalySee National Library of Medicine
This was a 20 patient study. 10 patients were treated and 10 were given a placebo. Liver enzyme levels were reduced by 20-30% on average with just one week of usage.In conclusion, IdB 1016 (Silybin Phytosome) seems to improve the biochemical signs of hepatocellular necrosis and/or increases cellular permeability in patients affected by chronic active hepatitis.
Clinical Study – Therapeutic effect of Silybin Phytosome in chronic liver disease
Therapeutic and Antilipoperoxidant Effects of Silibyn-Phosphatidylcholine Complex (Silybin Phytosome) in Chronic Liver Disease:
Preliminary Results
S. Moscarella1, A. Giusti1, F. Marra1, C. Marena2, M. Lampertico2, P. Relli1, P. Gentilini1 and G. Buzzelli1
Institute of Clinica Medica II, University of Florence, Florence, Italy and 2Medical Department, Inverna della Beffa, Milan, Italy
Journal not listed on Medline
Eight patients (two men and six women), with viral chronic active hepatitis were treated with (Silybin Phytosome) for two months. After treatment, serum malondialdehyde levels decreased by 36% (which indicates significantly less liver damage) and the quantitiative liver function evaluation increased by 15% (which is quite dramatic). A statistically significant reduction of transaminases was also seen. These results indicate (Silybin Phytosome) is effective in improving the biochemical and quantitative indices of hepatic function in patients with chronic active hepatitis.
Clinical Study – Direct comparison of Silybin Phytosome to standardized milk thistle extract
Comparative pharmacokinetics of silipide (Silybin Phytosome) and silymarin in rats
P. MORAZZONI1, A. MONTALBETTI1, S. MALANDRINO1 AND G. PIFFERI2
1 Inverni della Beffa Research and Development Laboratories, Milan Italy
2 Istituto di Chimica Farmaceutica, Univarea di Milano, Milan, Italy
See National Library of Medicine Citation
The plasma level profile and the biliary excretion of silybin, the main flavanolignan component of silymarin, were evaluated in rats after single equimolar oral doses of the silybin-phosphatidylcholine complex silipide (Laboratory code Idb 1016), (Silybin Phytosome), and of silymarin.
The introduction of this study states that the standardized extract of silymarin is widely used in Eurpoe for the treatment of liver disorders(1-3). It goes on to state the a problem with oral use of the main constituent of silymarin (silybin) is the low bioavailability of the compound, as demonstrated in studies with lab animals (4,5) and man (6).
Previous studies in rats (7), healthy volunteers (8) and patients with liver disease (9, 10) have shown that after oral intake of silipide (Silybin Phytosome), plasma silybin levels are several-fold higher than those measured after treatment with silymarin at equal doses in terms of silybin content.
This study showed the relative bioavailability of silipide (Silybin Phytosome) was nearly 10-fold higher than that of silymarin. Measured as silybin, the blood plasma peak for Silybin Phytosome was 1,004, whereas for silymarin it was 139.
Hruby K., Cosmos G., Fuhrmann M., Thaler H. (1983): Chemotherapy of Amanita phalloides poisoning with intravenous silybinin. Human Toxicol.2, 183-195.
Ferenci P., Dragosics B., Frank H., Benda L., Dittrich H., Meryn S. (1985): Randomized controlled trial of silymarin tratment in patinets with cirrhosis of the liver. J. Hepatol., 1, S229.
Ferenci P., Dragosics B., Dittrich H., et al (1989): Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol.,9, 105-113.
Morazzoni P., Magistretti M.J., Giachetti C., Zanolo G. (1992): Compartive bioavailability of silipide (Silybin Phytosome), a new flavanolignan complex, in rats. Eur. J. Drug Metab. Pharmacokinet., 17, 39-44.
Arcari M., Brambilla A., Brandt A., et al. (1992): Nuovo comlesso di incllusione tra la silybina e la ciclodrestrina: velocita di dissoluzione in vitro e assorbimento in vivo in confronto a fromulazioni tradizianali. Boll. Chim Farmaceutico, 131, 205-209.
Zanolo G., (1989): RBM Exp. N. 254, Inverni della Beffa SpA, data on file.
Morazzoni P., Malandrino S., Pifferi G. (1992) : Comparative bioavailability of a silybin-phosphatidylcholine complex (Silybin Phytosome) and silymarin in rats. In: Bres J., Panis G. (eds).
Barzaghi N., Crema F., Gatti G., Pifferi G., Perucca E. (1990): Pharmakinetic studies on Idb 1016, a silybin-phosphatidylcholine complex (Silybin Phytosome), in healthy human subjects. Eur. J. Drug Metab. Pharmacokinet., 15, 333-338.
Orlando R., Fragasso A., Lampertico M., Marena C. (1990): Silybin kinetics in patients with liver cirrhosis: a comparative study of a silybin-phospatidylcholine complex (Silybin Phytosome) And silymarin. Med. Sci Res. 18, 861-863.
Schandalik R., Gatti G., Perucca E. (1992): Pharmacokinetics in bile following administration of silipide (Silybin Phytosome) and silymarin in cholecystectomy patients. Arzneimittelforsch., 42 (II), 964-968
Clinical Study – Useful dosages of Silybin Phytosome
Randomized open study of the dose-effect relationship of a short course of IdB 1016 (Silybin Phytosome) in patients with viral or alcoholic hepatitis
A. Vailati, L.Aristia, E. Sozze, F. Milani, V. Inglese, PO. Calenda, P.A. Gossolo, E. Ascari
Department of Internal Medicine and Medical Therapy, 2nd Meidcal Clinic, University of Pavia, Italy
M. Lampertico, S. Comis, C. Marena
Medical Department, Inverni della Beffa, Via Ripamonti 99, 20041 Milan, Italy
Not on Medline
SUMMARY: A phase-II randomised open trial was performed to clinically evaluate the dose-response relationship to IdB 1016 (Silybin Phytosome), in patients with chronic hepatitis of either alcoholic or viral cause. The results suggest that treatment with (Silybin Phytosome) is of benefit in patients with viral or alcohol-induced hepatitis at the dose of 160 mg per day. But significant benefits were found at the dose of 240 mg per day. Still greater benefits were found with the dosage at 360 mg per day. All of these dosages are measured in silybin, which is 33% of the total in Silybin Phytosome, with phosphatidylcholine making up the balance. Therefore you must multiply by three to get the actual corresponding dosage of (Silybin Phytosome).
The lowest dose had some beneficial effect, but this study collected sufficient clinical and statistical evidence to indicate as minimum recommended dose the 240 mg per day (measured as silybin). Since there are no evidences of greater risks of adverse events with the 360 mg per day dose, whereas there are indications this may provide greater effects on more parameters than the 240 mg dose, the use of the highest dose may be considered in patients in need of either a faster onset or of a greater extent in the pharmacodynamic action of IdB 1060 (Silybin Phytosome).
Clinical Study – Considerably greater bioavailability of silybin as a component of Silybin Phytosome
Arzneimittelforschung 1992 Jul;42(7):964-8
Pharmacokinetics of silybin in bile following administration of silipide (Silybin Phytosome) and silymarin in cholecystectomy patients.
Schandalik R, Gatti G, Perucca E
Surgical Department, Regional Hospital, Braunau am Inn, Austria.
See National Library of Medicine Citation
These data indicate that the bioavailability of silybin is much greater after administration of silipide (Silybin Phytosome) than after administration of silymarin. This results in increased delivery of the compound to the liver, which represents the target organ for pharmacological action.
The biliary excretion of silybin, the main active component of silymarin, was evaluated by using a specific HPLC method in 9 cholecystectomy patients with T-tube drainage after intake of oral doses of silipide (Silybin Phytosome) (Silybin Phytosome) and of silymarin (120mg expressed as silybin equivalents)
After intake of silipide (Silybin Phytosome), the concentration of silybin in bile reached a peak within 4 h and declined thereafter with a mean time of about 10 h. After administration of silymarin, biliary silybin concentrations were several-fold lower than those observed after intake of silipide (Silybin Phytosome).
The bile collected after silymarin intake also contained considerable amounts of isosilybin (a silybin isomer) and very low levels of silydianin and silycristin. The amount of silybin recovered in bile in free and conjugated form within 48 h accounted for 11% of the dose after silipide (Silybin Phytosome) and for 3% of the dose after silymarin. Plasma silybin concentrations, determined in 3 subjects, were several-fold lower than those in bile after intake of silipide (Silybin Phytosome) and mostly undetectable after intake of silymarin.
Clinical Study – Increased oral bioavailability of Silybin Phytosome in humans
Eur J Drug Metab Pharmacokinet 1990 Oct-Dec;15(4):333-8
Pharmacokinetic studies on IdB 1016 (Silybin Phytosome), a silybin- phosphatidylcholine complex, in healthy human subjects.
Barzaghi N, Crema F, Gatti G, Pifferi G, Perucca E
Department of Medical Pharmacology, University of Pavia, Italy.
See National Library of Medicine Citation
IdB 1016 (Silybin Phytosome) is a complex of silybin (the main active component of silymarin) and phosphatidylcholine, which in animal models shows greater oral bioavailability and therefore greater pharmacological activity compared with pure silybin and silymarin. In order to assess its pharmacokinetic profile in man, plasma silybin levels were determined after administration of single oral doses of IdB 1016 (Silybin Phytosome) and silymarin (equivalent to 360 mg silybin) to 9 healthy volunteers. Although absorption was rapid with both preparations, the bioavailability of IdB 1016 (Silybin Phytosome) was much greater than that of silymarin, as indicated by higher plasma silybin levels at all sampling times after intake of the complex. Regardless of the preparation used, the terminal half-life was relatively short (generally less than 4 h).
In a subsequent study, 9 healthy volunteers received IdB 1016 (120 mg b.i.d., expressed as silybin equivalents) for 8 consecutive days. The plasma silybin level profiles and kinetic parameters on day 1 were similar to those determined on day 8. Most of the silybin present in the systemic circulation was in conjugated form.
Less than 3% of the administered dose was accounted for by urinary recovery of free plus conjugated silybin, a significant proportion of the dose probably being excreted in the bile. It is concluded that complexation with phosphatidylcholine in IdB 1016 (Silybin Phytosome) greatly increases the oral bioavailability of silybin, probably by facilitating its passage across the gastrointestinal mucosa.
Publication Types:
* Clinical trial
* Randomized controlled trial
PMID: 2088770, UI: 91209372
Clinical Study – Effect of Silybin Phytosome on cirrhotic patients
Med. Sci Res., 1991′ 19, 827-828
Pharacokinetic studies of silybin-phosphatidylcholine complex (Silybin Phytosome) in liver cirrhosis after multiple doses
Rocco Orlando, Annalisa Fragasso, Mario Lampertico1 and Carlo Marena1
Institute of Internal Medicine, University of Padua, Italy, and 1Medical Department, Inverni della Beffa, Milan, Italy
Journal not found on Medline
Introduction: Silybin is the main active component of silymarin, a standardized extract from the seeds of Silybum marianum Gaertn. (Silybin Phytosome) is a complex between silybin and soybean phosphatidylcholine, which exerts protective effects against hepatic lesions induced by toxic agents such as alcohol and phalloidin (1,2).
The data obtained in cirrhotic patients after multiple doses of IdB 1016 (Silybin Phytosome) did not show significant differences from those reported after a single dose. They clearly show that the drug does not accumulate in cirrhotic patients.
Furthermore the results indicate no significant difference from healthy control subjects.
It is therefore posited that this substance can be safely employed in long-term treatment of chronic liver patients.
1. Ferenci, P., Dragosics, B., Frank, H. et al. 1985. Abstr. J. Hepatol., 1, S229
2. Salmi, H.A. and Sarna, S. 1982, Scand. J. Gastroenterol., 17, 517-521
Clinical Study – Liver damage control properties of Silybin Phytosome
Japan. J. Pharmacol. 60, 315-321 (1992)
Protective Activity of Silipide (Silybin Phytosome) on Liver Damage in Rodents
Marisa Conti, Salvatore Malandrino and Maria Jose Magistretti
Inverni della Beffa Research and Development Laboratiories, via Ripamonti 99, Milan Italy
See National Library of Medicine Citation
The aim of this study was to assess the effect of the silipide (Silybin Phytosome) against liver damage induced by different agents.
The results of this study showed a significant and dose related protective activity against liver damage induced by toxic agents with different mechanisms of action. One of the poisons tested against was galactosomine which mimics hepatitis in its effect on the liver.
The constituents of silipide (Silybin Phytosome), on their own, were devoid of any significant activity at doses equivalent to those contained in the active doses of silipide (Silybin Phytosome).
The oral efficacy of silipide (Silybin Phytosome) and the lack of activity of the uncomplexed silybin indicate that silybin reaches active concentrations at the site of action only when administered as a complex. (1, 2)
Favorable results of preliminary clinical trials (3,4) indicate that silipide (Silybin Phytosome) can be considered as a promising new therapeutic agent for the treatment of hepatic disorders.
Morazzoni, P, Magistretti, M.J., Giachetti, C. and Zanolo, G.: Comparative bioavailability of IdB 1016 (Silybin Phytosome), a new flavanolignan complex, in rats. Eur. J. Drug Metab. Parmacokinet. 17, 39-44 (1992)
Barzaghi, N., Crema, F., Gatti, G., Pifferi, G. and Perucca, E.: Pharmacokinetic studies on IdB 1016, a silybiin-phosphatidylcholine complex, in healthy human subjects. Eur. J. Drug Metab. Pharmacokinet. 15, 333-338 (1990)
Marena, C. And Lampertico, M.: Preliminary clinical development of silipide (Silybin Phytosome): a new complex of silybin in toxic liver disorders. Planta Med. 57, A124-A125 (1991)
Buzzelli, G., Mascarella, S., Giusti, A., Marra, F., Pifferi, G., Marena, C. and Gentilini, P.: Free radical scavenging effects of silybin-phosphatidylcholine (Silybin Phytosome) in pationes with chronic liver disease, preliminary results. Abstracts of 26th meeting “European Association for the Study of the Liver”, September 1991, Palma de Mallorca (Spain), S103 (1991)
Clinical Study – Free radical scavenging properties of Silybin Phytosome
Free Rad. Res. Comms., Vol. 11, Nos. 1-3, pp.109-115
Studies on the Antioxidant and Free Radical Scavenging Properties of IdB 1016 (Silybin Phytosome) A New Flavanolignan Complex
A. Comoglio, G. Leonarduzzi, R. Carini, D. Busolin, H. Basaga*, E. Albano, A. Tomasi**, G. Poli, P. Morazzoni***, and M.J. Magistretti
*Department of Experimental Medicine and Oncology, University of Turin, **Institute of General Pathology, University of Modena and ***Inverni della Beffa Research and Development Laboratories, Milan, Italy
See National Library of Medicine Citation
Flavonoids are a wide group of plant-derived compounds which have been demonstrated to have antihepatotoxic effects. Among the flavonoids the purified extract of Silybum marianum (silymarin) and its main active constituent silybin have received substantial attention because of their antioxidant properties.
Nonetheless, the possible application of these flavonoids in therapy is hampered by their low solubility in water and by the poor enteral absorption. In order to increase absorption silybin has been complexed on a molecular level with phosphatidylcholine to create IdB 1016 (Silybin Phytosome). After oral administration of IdB 1016 (Silybin Phytosome) to rats the plasma levels of silybin were found to be significantly higher than those present after the administration of either uncomplexed silybin or silymarin to humans (1).
In conclusion of this study it was found that IdB 1016 (Silybin Phytosome) is able to reach intracellular sites at concentrations which make it effective as an antioxidant. It was also proven to target the liver preferentially. Also, the fact that it is capable of scavenging free radicals and inhibit lipid peroxidation suggests that this compound may be potentially useful to protect against free radical-mediated toxic damage.
1. N. Barzaghi, G. Perucca, G. Pifferi and A. Crema, (1989) Single-dose pharmacokinetic study of IdB 1016 (Silybin Phytosome), a new flavanolignan complex in man. European Journal of Clinical Pharmacology, 36 (suppl.), A110
Clinical Study – Effect of silimarin on lipid peroxidation
Pharmacological Research, Vol. 25, No. 2, 1992
Effect of the Flavanolignans of Silybum marianum L. on Lipid Peroxidation in Rat Liver Microsomes and Freshly Isolated Hepatocytes
ENRICA BOSISIO, CINZIA BENELLI AND ONDINA PIROLA
Institute of Pharmacological Sciences, Faculty of Pharmacy, University of Milan, Italy
See National Library of Medicine Citation
This study examines the effect of several flavonolignans (silicristin, silidianin, silybin and isosilybin) present in silymarin, the extract of Silybum marianum fruits, was tested on lipid peroxidation in rat liver microsomes and freshly isolated hepatocytes.
The study shows the antioxidant activity of the constituents in silymarin, with silybin proving to be the most valuable.
Clinical Study – Silybin Phytosome counteracts hepatotoxic effects
Biochemical Pharmacology, Vol. 43, No. 10, pp 2111-2115, (1992)
Lipid Peroxidation and Irreversible Damage in the Rat Hepatocyte Model
Protection by The silybin-phosopholipid Complex IdB 1016
Rita Carini, Adriana Comoglio, Emanuele Albano and Giuseppe Poli*
Department of Experimental Medicine and Oncology, University of Turin, CNR Centre of Immunogenetics and Experimental Oncology, Corso Raffaello 30, Torino, Italy
See National Library of Medicine Citation
Abstract-IdB 1016 (Silybin Phytosome) is a new silybin-phospholipid complex which is more bioavailable than the flavonoid silybin itself and displays free radical scavenging and antioxidant properties in liver microsomes. IdB 1016 (Silybin Phytosome) at the concentration which completely prevented MDA formation also protected isolated hepatocytes against toxicity. Similar protection was also obtained in hepatocytes prepared in vivo with IdB 1016 (Silybin Phytosome) while rat supplementation with pure silybin was totally inefficient. These results indicate IdB 1016 (Silybin Phytosome) as being a potentially useful protective agent against free radical-mediated toxic liver injury.
Moreover, it was demonstrated here for the first time that IdB 1016 (Silybin Phytosome) can counteract the hepatotoxic effect of several different compounds acting through oxidative mechanisms, indicating the potential usefulness of the compound as a hepatoprotective agent.
Clinical Study – Antioxidant activity of Silybin Phytosome against alcohol
Biochemical Pharmacology, Vol. 50 No. 8, pp. 1313-1316, 1995
Scavenging Effect of Silipide (Siliphos®), a new Silybin-phospholipid Complex, on Ethanol-Derived Free Radicals
A. Comoglio, A. Tomasi*, S. Malandrino**, G. Poli*** and E. Albano****
*Departments of Experimental Medicine and Oncology and Medical Sciences, University of Torino; ***C.N.R. Centre of Immunogenetics and Experimental Oncology, Torino; **Indena S.p.A. Milano and *Institut of General Pathology, University of Modena, Italy
See National Library of Medicine Citation
The results of this study indicate that (Silybin Phytosome) effectively scavenges hydroxhethyl radicals. However, this effect is lost when the same amount of pure silybin is administered instead. The ability of Silipide (Silybin Phytosome) to scavenge ethanol-derived radicals along with its antioxidant activity suggests that this drug might be potentially useful in counteracting free radical-mediated injuries involved in the development of liver damage caused by alcohol abuse.
Clinical Study – Tolerablility and effectiveness of Silybin Phytosome
Planta Med, 57 Supplement Issue 2, 1991
Preliminary Clinical Development of Silipide (Silybin Phytosome), A New Complex of Silybin, in Toxic Liver Disorders.
C. Marena and M. Lampertico
Medical Department, Inverni Della Beffa S.p.A., Milan, Italy
This study was conducted as a follow up to a study that confirmed excellent tolerablility at dosages up to 360 mg (as silybin equivalents) three times a day for three weeks. It was part of a comprehensive program of clinical investigations to evaluate the effect of the compound in patients with a variety of liver disorders.
This report is based on the preliminary data for 232 patients with liver disorders (including alcoholic and viral hepatitis) treated with IdB 1016 (Silybin Phytosome) capsules, 120 mg, two or three times a day for periods up to 120 days. Control subjects were also studied. 49 were treated with the commercially available extract (standardized to 70% silymarin). This standardized extract is known as Legalon in Europe and Thisilyn in the United States. 117 untreated or placebo treated were also studied. Evaluation was based mostly on biochemical markers.
The findings of the study confirm the effectiveness of silybin in restoring hepatic function in conditions associated with toxic or infectious liver damage. IdB 1016 (Silybin Phytosome) provides a chemically modified, well defined, more bioavailable formulation which exhibits greater clinical efficacy compared with the conventional standardized milk thistle extract (Legalon/Thisilyn).
Clinical Study – Liver protection potential of Silybin Phytosome
Planta Med, 57 Supplement Issue 2, 1991
Free Radic Res Commun 1990;11(1-3):109-15
Studies on the antioxidant and free radical scavenging properties of IdB 1016 (Silybin Phytosome) (Silybin Phytosome) a new flavanolignan complex.
Comoglio A, Leonarduzzi G, Carini R, Busolin D, Basaga H, Albano E, Tomasi A, Poli G, Morazzoni P, Magistretti MJ
See National Library of Medicine Citation
Department of Experimental Medicine and Oncology, University of Turin, Italy.
Silybin has been complexed in 1:1 ratio with phosphatidyl choline to give IdB 1016 (Silybin Phytosome) in order to increase its bioavailability. The antioxidant and free radical scavenger action of this new form of silybin has been evaluated. One hour after the intragastric administration to rats of IdB 1016 (Silybin Phytosome) IdB decreased by about 40% the lipid peroxidation induced in microsomes by NADPH, CCl4 and cumene hydroperoxide, probably by acting on lipid derived radicals.
Spin trapping experiments showed, in fact, that the complexed form of silybin was able to scavenge lipid dienyl radicals generated in the microsomal membranes. In addition, IdB 1016 (Silybin Phytosome) was also found to interact with free radical intermediates produced during the metabolic activation of carbon tetrachloride and methylhydrazine.
These effects indicate IdB 1016 (Silybin Phytosome) as a potentially protective agent against free radical-mediated toxic damage.
PMID: 2074043, UI: 91161019
Clinical Study – Comparative bioavailability of Silybin Phytosome vs. silybin
European Journal of Drug Metabolism and PharmacoKinetics, 1992, Vol. 17, No. 1, pp.39-44
Silybin Phytosome versus Silybin
Comparative bioavailability of IbD 1016 (Silybin Phytosome), a new flavanolignan complex, in rats
P. Morazzoni1, M.J. Magistretti1, C. GIACHETTI2 and G. ZANOLO2
1 Inverni della Beffa Rasearch and Development Laboratories, Milan, Italy
2 “Antoine Marxer” Institute for Biomedical Research, Ivrea, Italy
See National Library of Medicine Citation
The results of this study indicate a superior bioavailabilty of silybin administered orally as IbD 1016 (Silybin Phytosome). This was due mainly to an impressive increase in gastrointestinal absorbtion.
It also clearly confirmed that pure silybin has a very low bioavailability; indeed, administered by oral route it does not result in detectable plasma levels of either unmodified or total silybin.
Clinical Study – Liver protective activity
Department of Biochemistry
Faculty of Medicine
National University of Singapore
3rd International Symposium on Flavonoids in Biology & Medicine
November 1989
Liver Protective Activity of IdB 1016 (Silybin Phytosome) A new Flavanolignan Complex
M. Conti, S. Malandrino, M.J. Magistretti*
Inverni della Beffa Research and Development Laboratories, Milan, Italy
No reference on Medline
IThis substance was tested against its constituents (Silybin and Phosphotidylcholine) for protection against liver poisoning in lab rats. IdB 1016 (Silybin Phytosome) was found to reduce liver cell damage indicators from one poison tested by 43%. For another poison it reduced liver necrosis from 68% in poisoned controls to 5% in treated animals.
The compound was consistently more active than either Silybin or Phosphatidylcholine alone. These results may be explained by the improved bioavailablity of IdB 1016 (Silybin Phytosome)